Calreticulin, encoded by the CALR gene, is a Ca2+ binding protein that has been described in many cancer research studies. CALR mutations, mainly insertion/deletion mutations in its exon 9, have been found in majority of essential thrombocythemia (ET) and primary myelofibrosis (PMF) patients who do not have JAK2 and MPL mutations1,2. But CALR mutations were not reported in patients with polycythemia vera (PV). Studies have shown that CALR mutations are associated with lower leukocyte, higher platelet count and better survival of ET and PMF patients3-5.
Thus, CALR mutation analysis will provide clinically important information for patients who are suspected to have a myeloproliferative disorder. It assists the accurate diagnosis of myeloproliferative neoplasms and provide prognostic information.
Assay Advantages:
- Uses less clinical samples – works for small samples such as fine needle aspiration (FNA)
- Detects low level (1-5%) mutation allele frequency
- Results in 2 hours with sequencing-like accuracy
Target Mutations All insertion/deletion in codons 360 to 386 of CALR exon 9
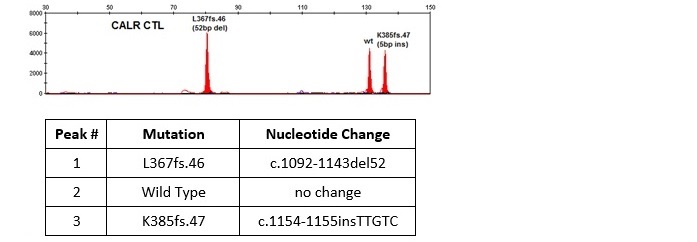
- most common deletion - L367fs*46 (c.1092_1143del52)
- most common insertion - K385fs*47 (c.1154_1155ins TTGTC)
Assay Sensitivity Detects 1-5% CALR insertion/deletion mutations in wild type background
Assay Format One tube test
Assay workflow Simple two-step protocol: PCR and Capillary Electrophoresis
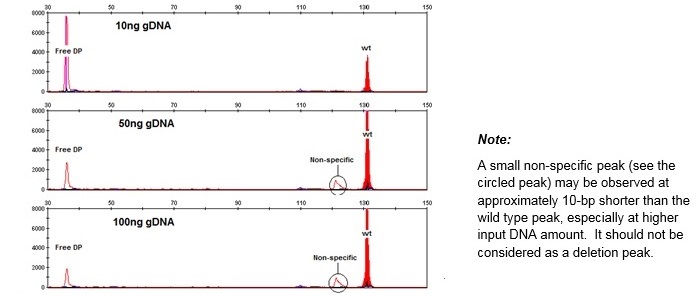
Sample required 10-100 ng DNA
Assay Platform Capillary sequencer ABI 3100, 3700, 3130, 3500
Order Information
| Cat. No. | Product Name | Target Mutations | Size | Documents |
| GP20 | CALR mutation analysis reagents | Insertions/Deletions in exon 9 | 32 rxn | Manual |
For research use only. Not for use in diagnostic procedures.
DATA
Example results of CALR controls with two most common mutations in exon 9:
Example results of genomic DNA titration experiments:
Related Products
- FFPE Sample DNA Extraction– high yield DNA in 1 hour, 99% PCR success rate
- Mutation Analysis Reagents for Oncology – single platform for any somatic mutations
- Mutation Analysis Reagents for Pharmacogenetics – single platform for any genotyping
References
- Klampfl T et al. (2013). Somatic mutation of calreticulin in myeloproliferative neoplasms. New Engl J Med 369:2379.
- Nangalia J et al. (2013). Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. New Engl J Med 369:2391.
- Rumi E et al. (2014). JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood 123:1544.
- Rotunno G et al. (2014). Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood 123:1552.
- Teferri A et al. (2014). CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons. Leukemia 28:1472.
